- Home
- Herbal Remedies
- Horopito
Is Horopito an Antifungal and Antibacterial Agent?
Posted 6/15/2025
Written By Pharmaceutical Scientist Dr. Harmeet Kaur, PhD
This article talks about horopito's effects on yeasts, bacteria and its possible safety issues and side effects.
The Horopito (Pseudowintera colorata and Pseudowintera axillaris), a unique flowering plant also known as Red Horopito, Winter’s bark, and New Zealand Pepper tree, is a pre-historic shrub endemic to many parts of New Zealand and belongs to the Winteraceae family. It is one of the oldest flowering plants which has survived 65 million years because of the superb natural chemical defense system in its leaves.

The leaves red coloration give protection against harsh ultraviolet radiation, and the pungent taste is a deterrent to insects and animals. Horopito has an extensive history of medicinal use internally as well as topically as an antiseptic, analgesic and quinine substitute for chronic diarrhea and stomach pain by New Zealand’s indigenous Maori population. Traditionally it is also used in colds and asthma, chest infections, and topical applications for a range of skin conditions. Recently, it has been used topically for bacterial and fungal infections, including Candida albicans, and internal preparations prescribed mainly by medical herbalists, particularly for digestive and respiratory tract infections [1,2].
Horopito's Active Compounds
The main active constituent present in horopito is a bicyclic sesquiterpene dialdehyde, polygodial, found primarily in P. colorata, and also responsible for the horopito hot peppery flavour while other dialdehydes like 9-Deoxymuxigadial are probably to be involved in the therapeutic actions of P. axillaris. The red color of the leaves enriched with anthocyanins, flavonols, and dihydroflavonols all of which are known to have potent antioxidant activity. Other active constituents present are tannins and essential oil components, such as eugenol, pinenes, limones, humulene and the flavonoids quercetin, luteolin and proanthocyanidins [3,4].
Role of Horopito as an Antifungal Agent
In 1982, Professor J.R.L Walker at the University of Canterbury in New Zealand, surprisingly noticed anti-fungal property of sesquiterpene dialdehyde, polygodial from horopito leaves against Candida albicans and found it very effective in comparison with the standard drug amphotericin B. The active constituents especially polygodial suppressed the growth of C. albicans from day one after incubation, while amphotericin B required three to four days.
Polygodial has also demonstrated antifungal activity towards Candida utilis, Candida krusei, Saccharomyces cerevisiae, Cryptococcus neoformans, and also other filamentous fungi like Trichophyton ruburum, Trichophyton mentagraphytes, and Pencillium marneffe.
The various susceptibility-testing conditions like incubation temperature, medium type, inoculum size and medium pH had little effect on the antifungal activity of the polygodial while its activity is significantly enhanced under acidic conditions. In the human host, the fungal environments such as the mouth, skin, and vagina often are acidic, and fungal colonization usually creates a microenvironment with even lower pH. In such conditions, polygodial has anticipated as an active antifungal agent [5,6].
Further, the polygodial has not shown any haemolytic activity (rupture of red blood cells) unlike Amphotericin B, and its mode of action is different from that of existing antifungal drugs. The polygodial exhibits synergistic fungicidal properties with some preservatives used in the food industry. On combination with half the minimum fungicidal concentration of polygodial with sorbic acid and benzoic acid commonly used preservatives, the fungicidal effects against S. cerevisiae have enhanced by 64-fold and 400-fold respectively [7].
With EDTA, another food preservative the polygodial exerts a synergistic effect, presumably by facilitating its transport into yeast cells. The polygodial can also control Zygosaccharomyces baillii at very low concentrations which is a spoilage yeast that can survive in acidic media with ethanol even [8].
Further, a combination of milled horopito leaf with milled anise seed (containing anethole) showed synergistic effect against budding yeast, Saccharomyces cerevisiae, and a human opportunistic pathogenic yeast, Candida albicans and significantly improved the chronic intestinal candidiasis in the patients [9].
In a study, Kumari et al. further assessed the clinical efficacy of a phytochemical combination K-712 (containing 10 mg of oleoresin from P. colorata having 30 % of polygodial together with trace amounts of Olea europea) in 82 women participants with recurrent vulvovaginal candidiasis (RVVC) in comparison with azole drug itroconazole for total 12 months. The K-712 was proved to be as good as itraconazole during first 6-month treatment, but afterwards 6-month observation period, the mycological success rate was significantly increased by phytocombination due to the reduced number of relapses and probably of the growth of azole-resistant species where the itraconazole group showed significantly more relapses [10].
Role of Horopito as an Antibacterial Agent
In earlier reports, no or little antibacterial activity has reported for the polygodial as it was tested against only a few selected bacterial strains, at the highest concentration of 100 µg/mL [11].
Traditionally, Maori people used horopito leaves as a poultice for wounds and the sap was used for the infection caused by Neisseria gonorrhoea and various skin diseases [12].
Based on the surfactant concept found mainly against S. cerevisiae, polygodial can be expected to possess a broad spectrum due to its nonspecific surfactant like mechanism. Recently, the polygodial’s antibacterial activity has been reexamined against several bacteria. Polygodial demonstrated moderate antibacterial activity towards Gram-positive bacteria including Staphylococcus aureus (seven strains including two methicillin-resistant), Bacillus subtilis, and Gram-negative bacteria including Escherichia coli, Enterobacter aerogenes, Proteus vulgaris, and Salmonella choleraesuis with minimum bactericidal concentrations (MBCs) ranging from 100 to 400 μg/mL. The polygodial has been found to possess a broad antimicrobial spectrum, except Pseudomonas aeruginosa. The time-kill assay has shown the bactericidal action of polygodial against S. choleraesuis and B. subtilis, though, there was a difference in its bactericidal action against food-borne S. choleraesuis and endospore-forming B. subtilis [13].
The P. colorata hydro-alcoholic leaf extract evaluated for inhibition of the growth of bacterial triggers for autoimmune disorders such as P. mirabilis (linked with rheumatoid arthritis), K. pneumoniae (responsible for ankylosing spondylitis), A. baylyi and P. aeruginosa (linked with multiple sclerosis). The extract was found potent inhibitor of A. baylyi, with MIC values as low as 427 µg/mL. P. vulgaris and P. mirabilis were also found highly prone to the P. colorata hydro-alcoholic leaf extract, with MIC in the range of 800-900 µg/mL. The extract has shown moderate activity against all other bacterial strains tested with MIC >1000 µg/mL. The extract was found to be nontoxic to the healthy cells as confirmed by the Artemia franciscana nauplii bioassay. The growth inhibitory bioactivity and the lack of toxicity of P. colorata leaf extracts in autoimmune inflammatory diseases triggered by various bacteria have indicated its potential in the prevention and treatment of these diseases in genetically susceptible individuals [14].
Horopito's Mechanism of Action
The underlying antifungal action of polygodial comes from its ability to act as a surfactant at the lipid-protein interface of integral proteins, like transport proteins and/or ion channels, denaturing their functional conformation, and then inhibiting various cellular functions. As a surfactant, the ability to form a pyrrole derivative with a primary amine group of phosphatidylserine (PS) and phosphatidylethanolamine (PE) in the outer monolayer of the cell membrane of microorganisms is likely in part, an initial step of the antifungal action of polygodial.
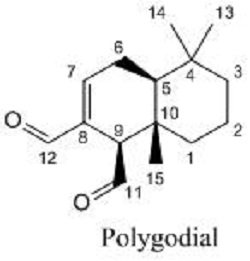
It appears that forming the pyrrole is polygodial’s specific dialdehyde structural function. Once a pyrrole is formed, the hydrophobic decalin portion may enter into the lipid bilayer and change the fluidity of the plasma membrane or disrupt the balance, causing fungal lysis and subsequent fatal loss of intracellular materials. In addition to the surfactant concept, polygodial may enter into the cells through pores derived from membrane damage [15].
It also likely permeates in part by passive diffusion across the plasma membrane. Mostly micro-organisms also protect themselves against superoxide (O2−) and hydrogen peroxide in various ways and some of the most ubiquitous systems include glutathione [16]. Polygodial has also stated to cause depletion of cytoplasmic and mitochondrial glutathione which functions by eliminating reactive oxygen species [17].
The activity of polygodial is initially considered to be due to the aldehyde groups, especially the one at C8, but later on, it has been found that the double bond present (between C7 and C8 in the drimane skeleton) is an essential structural feature. Polygodial also produces amounts of potassium leakage from yeast cells, similar to those produced by amphotericin B and miconazole.
Horopito's Side Effects & Toxicity
Contrary to compounds of a similar structure with potent antimicrobial action, polygodial exhibits the least cytotoxicity and is non-mutagenic confirmed by Ames and V79/HGPRT assay. Even though there is no evidence of teratogenicity, as a precaution, horopito has not been suggested during pregnancy or lactation. Horopito works rapidly against C. albicans in the digestive tract. So, in the first few days of therapy by those with Candida overgrowth, a Herxheimer reaction to dead candida cells is sometimes experienced which is characterized by nauseous feeling and headache, both of which usually are mild and transient [18,19].
Horopito Conclusion
To conclude, despite continuing advances in antifungal and antibacterial treatment, there is an increasing demand for natural alternatives that are effective, safe and convenient. Horopito is one of such natural compound. In vitro studies have revealed that horopito has an excellent potential of antifungal as well as antibacterial activity in terms of effectiveness and spectrum of action while being devoid of any significant toxicity. Moreover, this natural product has also anticipated being a promising compound for the development of therapeutic regimens, acting through a synergistic effect.
About the Author

Dr. Harmeet Kaur received her Bachelors in Pharmacy from Guru Nanak Dev University in Amritsar, India in 2000. Guru Nanak Dev University is a state owned university with an "A" grade nationally.
Dr. Kaur received her Masters in Medicinal Chemistry from the National Institute of Pharmaceutical Education and Research in 2002.
In 2015 Dr. Kaur was awarded her Ph.D in Pharmaceutical Sciences from Maharshi Dayanad University in Rohtak, India.
Dr. Kaur is presently a Senior Research Scientist at Maharshi Dayanand University in India.
Dr. Kaur has over 35 published Research papers concerning infectious diseases caused by yeasts, fungi, and bacteria using both prescription drugs and natural plant compounds. She has also performed many studies on cancer cells.
Of particular importance to us, is her multiple experiments performed on Candida albicans and pathogenic bacteria using natural compounds. Because of this experience, we are thrilled to have her on the Yeast Infection Advisor Team.
Back to Herbal Yeast Infection Remedies
Any questions about horopito or yeast infections in general, please contact us from the contact page of this website or talk to your doctor.
Dr. Kaur's Medical References
- 1. Rasmussen P. Pseudowintera spp.(Horopito): a monograph. Australian Journal of Herbal Medicine. 2014;26(4):150.
- 2. Vink W. Taxonomy in Winteraceae. Taxon. 1988;37(3):691-8.
- 3. Kubo I, Taniguchi M. Polygodial, an antifungal potentiator. Journal of Natural Products. 1988;51(1):22-29.
- 4. Larsen L. "A Literature Survey of the Constituents of Pseudowintera colorata." Dunedin, New Zealand: Crop and Food Research, Ltd., 2001.
- 5. McCallion RF, Cole AL, Walker JR, Blunt JW, Munro MH. Antibiotic substances from New Zealand plants. Planta medica. 1982;44(03):134-8.
- 6. Calder VL, Cole AL, Walker JR. Antibiotic compounds from New Zealand plants. III: a survey of some New Zealand plants for antibiotic substances. Journal of the Royal Society of New Zealand. 1986;16(2):169-81.
- 7. Kubo I, Lee SH. Potentiation of antifungal activity of sorbic acid. Journal of Agricultural and Food Chemistry. 1998;46(10):4052-5.
- 8. Fujita KI, Kubo I. Naturally occurring antifungal agents against Zygosaccharomyces bailii and their synergism. Journal of Agricultural and Food Chemistry. 2005 29;53(13):5187-91.
- 9. Fujita KI, Tatsumi M, Ogita A, Kubo I, Tanaka T. Anethole induces apoptotic cell death accompanied by reactive oxygen species production and DNA fragmentation in A spergillus fumigatus and S accharomyces cerevisiae. The FEBS Journal. 2014 ;281(4):1304-13.
- 10. Kumari A, Bishier MP, Naito Y, Sharma A, Solimene U, Jain S, Yadaw H, Minelli E, Tomella C, Marotta F. Protective effect of an oral natural phytonutrient in recurrent vulvovaginal candidiasis: a 12-month study. Journal of Biological Regulators & Homeostatic Agents. 2011;25(4):543-51.
- 11. Taniguchi M, Adachi T, Oi S, Kimura A, Katsumura S, Isoe S, Kubo I. Structure-activity relationship of the Warburgia sesquiterpene dialdehydes. Agricultural and biological chemistry. 1984;48(1):73-8.
- 12. Best E. Māori Medical Lore. The Journal of the Polynesian Society.1905; 14(1), 1-23.
- 13. Kubo I, Fujita KI, Lee SH, Ha TJ. Antibacterial activity of polygodial. Phytotherapy Research.2005;19(12):1013-7.
- 14. Barillot C, Davis C, Cock IE. Pseudowintera colorata (Raoul) dandy hydro-alcohol leaf extract inhibits bacterial triggers of some autoimmune inflammatory diseases. Pharmacognosy Communications. 2017;7(4):164-71.
- 15. Fujita KI, Kubo I. Multifunctional action of antifungal polygodial against Saccharomyces cerevisiae: involvement of pyrrole formation on cell surface in antifungal action. Bioorganic & medicinal chemistry. 2005;13(24):6742-7.
- 16. Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. The Annual Review of Biochemistry. 2008 ;77:755-76.
- 17. Kubo I, Fujita KI, Lee SH. Antifungal mechanism of polygodial. Journal of Agricultural and Food Chemistry. 2001 Mar 19;49(3):1607-11.
- 18. Morales P, Andersson M, Lewan L, Sterner O. Structure-activity relationships for unsaturated dialdehydes. 6. The mutagenic activity of 11 compounds in the V79/HGPRT assay. Mutational Research. 1992;268(2):315-321.
- 19. Forsby A, Andersson M, Lewan L, Sterner O. The cytotoxicity of 22 sesquiterpenoid unsaturated dialdehydes, as determined by the neutral red absorption assay and by protein determination. Toxicology In Vitro. 1991;5(1):9-
Home Privacy Policy Copyright Policy Disclosure Policy Doctors Store
Copyright © 2003 - 2025. All Rights Reserved under USC Title 17. Do not copy
content from the pages of this website without our expressed written consent.
To do so is Plagiarism, Not Fair Use, is Illegal, and a violation of the
The Digital Millennium Copyright Act of 1998.