- Home
- Herbal Remedies
- Clove
Clove As A Candida Yeast & AntiMicrobial Treatment
Posted 6/15/2025
Written By Pharmaceutical Scientist Dr. Harmeet Kaur, PhD
This article talks about Clove's effects on yeasts, bacteria, viruses, parasite infections and other health benefits along with possible safety issues and side effects.
Clove, also known as Syzygium (S.) aromaticum, belongs to the Myrtaceae family, is a dried flower bud that is indigenous to the Maluku islands in Indonesia but has recently been farmed in different places worldwide. The clove tree is composed of buds and leaves (the commercial part of the tree).
Excitingly, it is used commercially for many medicinal properties and in the perfume industry, and it is considered one of the spices that has gained much attention among the other spices and potentially used as preservatives in many foods, especially in meat processing, due to its antimicrobial and antioxidant properties and have replaced the chemical preservatives. The cloves have been used for centuries in the treatment of vomiting, flatulence, nausea, digestion problem, and as a stimulant for the nerves.
Cloves are used in Chinese and Indian traditional medicine as a stimulating and warming agent. In tropical Asia, cloves have been reported to relieve various microorganisms as scabies, cholera, malaria, and tuberculosis. Nowadays, several reports have confirmed the antifungal, antibacterial, antiparasitic, antiviral, and anticarcinogenic potential of this spice plant.
Clove essential oil (CEO) is used in the treatment of burns and wounds, and dentistry as a pain reliever as well as treating tooth infections and toothache. Additionally, its use has been documented in several industrial applications and is used widely in soaps, perfumes, and as a cleansing vehicle in histological work [1,2].
Chemical Composition of Clove
Approximately 27 species of clove have been found all over the world. Pharmacologically, clove has been a renowned source of phenolic compounds like, flavonoids, hydroxybenzoic acids, hydroxyphenyl propenes, hydroxycinnamic acids, and eugenol (C10H12O2)-which is the major bioactive molecule and hydrolyzable tannins like gallic acid that are found in high amounts in the fresh plant.
The flavonoids present in the clove are mainly kaempferol and quercetin along with phenolic acids like ferulic, caffeic, ellagic, and salicylic acids. Clove flower buds contain up to 18-20% of essential oil which is isolated by hydro-distillation and analyzed by Gas chromatography-mass spectroscopy (GC-MS)
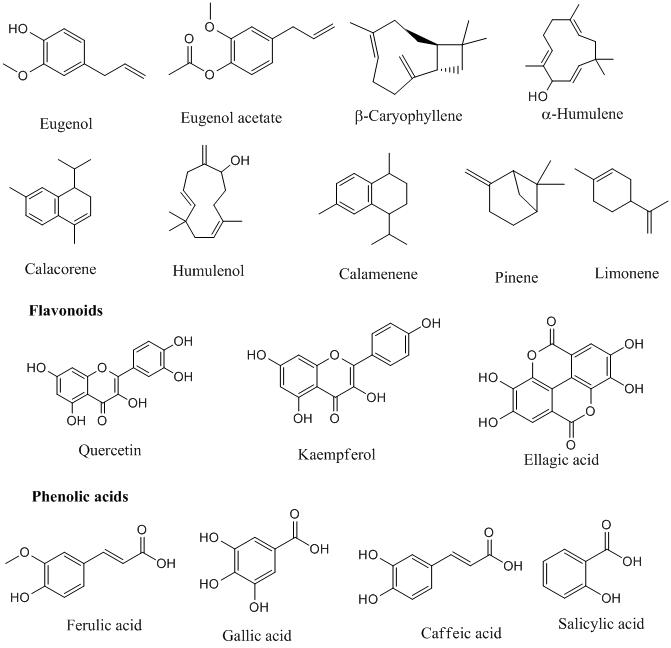
Clove oil is pale yellow or colorless with a distinct clove flavor and taste. The differences in clove essential oil content and composition are mainly influenced by several factors like variety, pre-treatments, agro-ecological conditions, and extraction processes. The clove essential oil demonstrates the presence of 36 components. The proportion of volatile components in oil varies from 95.16% to 99.91%, and the highest content of these volatile components is eugenol (48.2% to 50.22%). The other constituents present are eugenol acetate, β-caryophyllene, ethyl hexanoate, β-pinene, limonene, farnesol, 2-heptanone, α-humulene, calacorene, humulenol, calamenene, and some trace elements.
Eugenol was chemically transformed into two derivatives i.e. eugenol benzoate and eugenol acetate. The eugenol generally donates hydrogen ion and subsequently stabilize the phenoxy radical by resonance, which results in the formation of steady molecules that do not establish or increase oxidation.
Clove also revealed the presence of ash, crude fat, crude fiber, crude protein, moisture, and total soluble sugars content. The mineral analysis showed the presence of potassium (K), calcium (Ca), magnesium (Mg), phosphorus (P), and sodium (Na) in an appreciable amount [3,4].
Antibacterial Activity of Clove
The antibacterial activity of different clove extracts has been demonstrated against various pathogenic bacteria and food-borne pathogens including Campylobacter jejuni, Salmonella enteritidis, Salmonella typhimurium, Listeria monocytogenes, Vibrio vulnificuss, Escherichia coli, and Staphylococcus aureus. A study by Mytle et al testified that the growth rates of Listeria monocytogenes strains observed at 5 -15°C were expressively reduced by treatment with 1% and 2% clove oil [5].
Ogunwande et al found that the
essential oil of the fruit showed strong antibacterial activity
against S. aureus,
while the oil from leaves strongly inhibited the growth of B.
cereus, with a MIC of 39
µg/mL [6].
Fu et al.
as well as Palombo and Semple reported the antibacterial effect of
pure clove oil either alone or combined with rosemary oil towards
B. subtilis, P.
aeruginosa, S.
aureus, S.
epidermidis, Proteus
vulgaris, E.
coli,
and methicillin-resistant S.
aureus and
S. epidermidis
[7,8].
The coagulase-negative Staphylococci (CoNS) represent a hazard to hospital patients with poor immune responses and can survive a long time on medical devices. Hence the antibacterial action of clove oil was investigated against 32 multi-resistant S. epidermidis strains isolated from dialysis biomaterial using an agar disk diffusion assay. The clove oil has shown activity against this Gram-positive bacteria, demonstrating a clear zone of inhibition against the majority of the tested strains. Additionally, the highest level of activity was noticed against five strains of S.epidermidis (CIP106510, S27, S38, E13, and S23), with an inhibition zone of >16 mm. The oil was also found active against 26 strains of S. epidermidis isolated from dialysis fluids, three human pathogenic Gram-positive cocci, one Gram-positive Bacillus, and two Gram-negative Bacilli (diameter of inhibition zone: 11-15 mm). The clove essential oil also revealed antibacterial activity against a large number of methicillin-resistant S. aureus and S. epidermidis [9].
Jiazhang Qiu et al demonstrated that eugenol at the subinhibitory concentrations (16 µg/mL -128 µg/mL), dose-dependently decreased the hemolytic activity and the release of tumor necrosis factor-alpha (TNF-α) in Gram-positive bacterium S. aureus. Eugenol, also significantly decreased the production of Staphylococcal enterotoxin A (SEA), SEB, and reduced the expression of key enzyme hemolysin (which causes hemolysis). Hence eugenol has the potential of being used as a food additive due to its inhibitory effect on the growth of bacteria and suppressive effect on the production of exotoxins by S. aureus [10].
Sabahat Saeed et al conducted a study to investigate the potential of using aqueous infusion, decoction, and essential oil of clove as natural antibacterial agents against 100 isolates belonging to 10 different species of Gram-positive bacilli viz., Proteus mirabilis (6), Pseudomonas aeruginosa (10), Escherichia coli (36), Enterobacter aerogenes (5), Klebsiella pneumoniae (24), Klebsiella ozaenae (2), Shigella dysentriae (5), Serratia marcescens (4), Salmonella typhi (3), and Vibrio cholerae (5) by disc diffusion method. The decoction and aqueous infusion of clove shown maximum activity against P. aeruginosa with 10.86 mm and 10.43 mm zone of inhibition respectively. The essential oil of clove exhibited maximum activity against V. cholera with a 23.75 mm zone of inhibition. The bacterial strains such as K. pneumoniae, K. ozaenae, S. typhi, S. marcescens, V. cholera, and S. dysentriae were found resistant to aqueous infusion and decoction while essential oil had revealed a strong antimicrobial action against all tested bacterial isolates [11].
Leite et al assessed the effects of eugenol, α-pinene, and β-pinene, in inhibiting the growth of potential infectious endocarditis (inflammation of the endocardium) causing Gram-positive bacteria. S. epidermidis, S. aureus, Streptococcus pneumoniae, and S. pyogenes by solid medium diffusion method. These constituents demonstrated effectiveness in inhibiting all tested bacteria strains to present MIC values between 2.5 and 40 µL/mLwith the lowest MIC (2.5 and 5 µL/mL) exhibited by eugenol for the most bacteria strains [12].
Helicobacter pylori were subsequently found to be an important etiological agent for peptic ulcers, gastritis, and gastric malignancy. Ali et al tested eugenol and cinnamaldehyde against H. Pylori. These compounds have already been found to inhibit 10 different multidrug-resistant pathogenic bacteria such as Staphylococcus, Proteus, E. coli, Klebsiella, Pseudomonas, and Enterobacter isolated from human subjects. Eugenol and cinnamaldehyde completely inhibited all the strains (both sensitive & resistant) at a concentration of 2 μg/ml which is very less in comparison to other reported compounds such as garlic oil: 8-32 μg/ml allicins: 6-12 μg/ml, and ajoenes: 10-25 μg/ml.
These compounds also exhibited enhanced activity at low pH levels which may help them achieve their efficacy in an environment such as the human stomach. The eugenol and cinnamaldehyde inhibited H. pylori at 2 μg/ml concentration within 9 and 12 hours of incubation respectively while common antibiotic Amoxycillin inhibited H. pylori at the 12th hour of incubation itself. Furthermore, the H. pylori did not develop any resistance to the eugenol and cinnamaldehyde even after 10 successive passages grown at sub-inhibitory concentrations (0.25 and 0.5 μg/ml) whereas the organism strains acquired resistance to clarithromycin and amoxicillin after 10 sequential passages. Also, these bioactive compounds had equal bactericidal activity against both the susceptible and resistant H. pylori strains [13].
Hemaiswarya et al studied the synergistic effect of the eugenol with ten different conventional hydrophilic and hydrophobic antibiotics against five different Gram-negative bacteria (E. coli, Proteus vulgaris, Enterobacter aerogenes, Pseudomonas aeruginosa, and Salmonella typhimurium) The combinations exhibited MIC decreased by a factor of 5-1000 times in comparison to the MIC of individual components. This synergistic effect is exhibited due to the membrane damaging nature of eugenol (50% of the bacterial membrane can be damaged by 1 mM of its concentration). Eugenol also enhanced the activities of Triton X-100, lysozyme, and SDS (sodium dodecyl sulfate) in damaging the bacterial cell membrane. The hydrophilic antibiotics such as β-lactam antibiotics and vancomycin having a negligible antibacterial activity also exhibited an enhanced antibacterial activity against these Gram-negative bacteria when pretreated with eugenol [14].
Alicyclobacillus acidoterrestris is aerobic, Gram-positive bacteria, generally found in soil, on the surface of fruits, and known to colonize on a variety of technical materials (nylon, stainless steel, glass, polyvinyl chloride (PVC), polystyrene) and form biofilms. It can also withstand harsh conditions, including a wide range of temperature (20-60°C) and pH (2.0-6.0) and. The effect of essential oil of clove on bacterial biofilms at PVC and glass surfaces under static and agitated culture conditions was investigated by Kunicka-Styczyńska et al using atomic force microscopy and the plate count method. The essential oil in 0.05% (v/v) caused a 25-65 % reduction of biofilms on the technical surfaces accompanied by significant changes in their morphology by a decrease in the biofilm: bacterial cell length, surface roughness, surface area difference, and height. The essential oil exerted its action by releasing EPS (extracellular polymeric substances (EPS) stabilizes the biofilm and provides an elevated level of bacterial protection against unfavorable conditions) from the biofilm and so persuades detachment of bacteria from the surface. Hence the clove oil may find application in the juice industry to hinder the development of A. acidoterrestris biofilms on production surfaces [15].
Cloves Mechanism of Action
The mechanism of action of eugenol and essential oil on the bacterial membrane of S. pyogenes, L. monocytogenes, Proteus vulgaris, and E. coli has been explored. Essential oils mainly destabilize the cellular architecture accompanied by the breakdown of membrane integrity and increased permeability resulting in disruption of many cellular processes, including membrane transport, energy production (membrane-coupled), and other metabolic regulatory activities.
The disruption of the cell membrane by essential oils may affect several key processes like nutrient processing, energy conversion processes, the secretion of growth regulators, and the synthesis of structural macromolecules.
The secretion of toxins by bacteria also prevented by modifications in the bacterial membrane owing to the influence of the essential oil constituents on the transmembrane transport process in the plasma membrane, limiting the release of toxins to the external environment.
The antimicrobial effect of essential oils is also associated with the disruption of proton pumps, reduced membrane potentials, and the depletion of ATP. Both eugenol and clove essential oil constitute phenolic compounds that can denature proteins and react with cell membrane phospholipids altering their permeability. On treatment with clove oil mainly three biological macromolecules (ATP, protein, and DNA) are mostly leaked with the reduction of two intracellular enzymes (alkaline phosphatase and β-galactosidase) activities.
The clove oil also affects the respiratory metabolism of L. monocytogenes by reducing the activity of three key enzymes (citrate synthase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase) in the tricarboxylic acid pathway. The results of UV absorption spectroscopy have shown that eugenol, can also change the structure of DNA via the formation of eugenol-DNA chimera [16, 17].
Antifungal Activity of Clove
The antifungal activity of the clove oil has been proved against a variety of molds and yeast such as Candida albicans, C. parapsilosis, C. krusei, Alternaria sp., Aspergillus niger, A. fumigatus, Penicillum sp., Microsporum gypseum, Microsporum canis, Trichophyton mentagrophytes, T. rubrum, and Epidermophyton floccosum.
The essential oil passes through the cell wall due to the lipophilic nature and damages the cytoplasmic membrane while disrupting various layers of fatty acids, polysaccharides, and phospholipids, eventually making them permeable.
The antifungal activity of clove oil has also been attributed to a decreased synthesis of ergosterol, the major component of the fungal cell wall responsible for maintaining cell function and integrity [18].
Clove oil in the range of 2.5-10.0% concentrations was also effective against food contaminants B. cereus, B. subtilis, Staphylococcus sp., E. coli, Rhizopus sp., Aspergillus sp. and Penicillium sp. Dermatophytes infections are caused by approx. 40 species of fungi, and are grouped into three genera; Microsporum, Trichophyton, and Epidermophyton and mostly include infections of nails, hair, and skin of both humans and animals.
Eman-Abdeen et al carried out the in vitro study to evaluate the antifungal activity of Clove oil at different dilutions (0, 10, 20, 50, and 100%) on the isolated fungi (Microsporum canis, Trichophyton mentagrophytes, Aspergillus flavus, and Candida albicans) by disc diffusion method and confirmed the results through the application of clove oil on cows affected with dermatophytosis. The results revealed that T. mentagrophytes and M. canis and were the most predominant isolates and clove oil exhibited high in vitro antifungal activity against tested isolated fungi especially against Dermatophytes sp. and efficiently as a topical treatment in cows with dermatophytosis.
The phenolic components, eugenol, and carvacrol are known to have fungicidal characteristics, including activity against fungi isolated from onychomycosis [19].
Reports have also demonstrated the antifungal potential of eugenol against C. albicans and Trichophyton mentagrophytes. Núñez et al. showed that the mixture of clove oleoresin dispersed in concentrated sugar solution produced a strong fungicidal effect against C. albicans and Trichophyton mentagrophytes by reducing fungi inoculum size.
The fungicidal activity of clove-sugar on C. albicans, was similar to commonly used disinfectants in hospitals, such as chloroxylenol and povidone-iodine. The scanning electron microscopy micrographs showed significant morphological damage with cellular deformity to Saccharomyces cerevisiae cells by clove oil [20].
Laila Muñoz et
al investigated that essential oil from clove reduced the growth of
Fusarium oxysporum to
40% and Aspergillus niger from
50% to 70%. The functional extracts (FEs) (addition of fatty
acid ethyl esters as extraction co-solvents) of clove and pepper,
mixed with ethyl decanoate (FEs-C10),
were found to be the best in vivo
combination for protecting the tomato fruit against both
phytopathogenic fungi [21].
Eugénia Pinto et al evaluated the antifungal activity of clove oil and eugenol against various pathogenic fungal strains such as five Candida clinical strains, isolated from recurrent cases of oral candidosis, and four ATCC type strains; five dermatophyte clinical strains Microsporum gypseum, Microsporum canis, Trichophyton rubrum, Trichophyton mentagrophytes and Epidermophyton floccosum isolated from nails and skin; one clinical strain of Aspergillus flavus isolated from bronchial secretions, and two Aspergillus ATCC type strains (A. fumigatus and A. niger). Candida parapsilosis ATCC 90018 and Candida krusei ATCC 6258 were used for quality control.
The essential oil was found active against all the tested strains by exhibiting very low MIC and MFC values. The highest activity was reported against five different species of dermatophytes, showing a MIC value of 0.16 μl ml−1. The MIC values against Aspergillus and Candida strains ranged from 0.32 to 0.64 μl ml−1. Germ tube formation, generally regarded as an important mechanism of pathogenicity of C. albicans was strongly inhibited by clove oil and eugenol and this effect started at concentrations lower than their MIC value (0.64 μl ml−1). Hence this study reveals the broad-spectrum antifungal activity of clove oil which inhibited not only dermatophytes, Aspergillus and Candida species (such as C. albicans, C. parapsilosis, and C. tropicalis ), but also fluconazole-resistant C. albicans isolates, C. glabrata, whose resistance is easily inducible and C. krusei, intrinsically resistant to fluconazole [22].
Kaur et al evaluated the antifungal and antioxidant potential of clove essential oil, its major compound, and its derivatives. The antifungal activity was screened against three pathogenic fungi (Helminthosporium oryzae, Fusarium moniliforme, and Rhizoctonia solani) and antioxidant potential was checked by DPPH and nitric oxide (NO) free radical scavenging assays respectively. Clove essential oil has shown moderate antifungal activity against all the three tested fungi. However eugenol benzoate, the main component present was most effective against Helminthosporium oryzae and Fusarium moniliforme and with ED50 values of 5.3 and 10.17 μg/ml respectively while eugenol acetate was found most effective against Rhizoctonia solani, with ED50 value of 11.76 μg/ml.
Clove essential oil exhibited high antioxidant potential with IC50 of 10.87-31.63 μg/ml. Hence, the derivatives of eugenol isolated from clove essential oil showed maximum antifungal potential while the antioxidant potential of clove essential oil was found to be maximum [23].
Cloves Antiviral Activity
Viruses are highly sensitive to the constituents of the essential oils, and monoterpenes, and phenylpropanoids, have shown in vitro antiviral activity. Hussein et al. found that clove extract was highly active at inhibiting replication of the hepatitis C virus (≥90% inhibition at 100 µg/mL) (24). Kurokawa et al. isolated and identified an anti-HSV compound, eugeniin (a type of ellagitannin), from the extracts of clove, which has been documented for its antiviral efficacy towards various herpes virus strains and the hepatitis C virus by inhibiting the viral DNA polymerase enzyme essential for the synthesis of the viral DNA [25].
Another research revealed the antiviral efficacy of clove aqueous extracts against herpes simplex virus type 1 (HSV-1) and influenza A virus when combined with acyclovir. The possible antimicrobial action for clove oil is attributed to eugenol which consists of about 85% to 92% of total clove oil content [26].
Cloves Antiparasitic Effects
Interestingly, several reports documented that eugenol isolated from clove extracts have shown potent trypanocidal as well as leishmanicidal efficacy against Trypanosoma cruzi, Leishmania amazonensis, L. donovani, L. major, and L. tropica. Additionally, eugenol showed a potential lethal efficacy against the growth and multiplication of various parasites including Fasciola gigantica, Giardia lamblia, Schistosoma mansoni, and Haemonchus contortus [27,28].
The eugenol in cloves is believed to help fight parasites in the intestines by dissolving the outer casing of the parasitic eggs. It's more effective antiparasitic combination with black walnut and wormwood, as the eugenol, makes it easier for the black walnut and wormwood to invade the eggs and break the lifecycle of the parasites [29].
Cloves Other Health Benefits
Clove is a well-known and important herbal remedy due to its broad pharmacological efficacy. Mostly dried flower buds, leaves, stems, and oils are used to make medicine. In traditional medicine, clove has been used in indigestion complaints, flatulence, diarrhea, intestinal gas, nausea, and vomiting.
Eugenol, the main component present in clove oil has been long known for its local anesthetic, analgesic, anti-inflammatory, and antibacterial effects.
Pharmacologically, clove oil has wide application as an antiseptic in oral diseases and for the treatment of toothaches, for pain control during dental work, topically pain relief from mouth and throat inflammation, along with athlete's foot [30, 31].
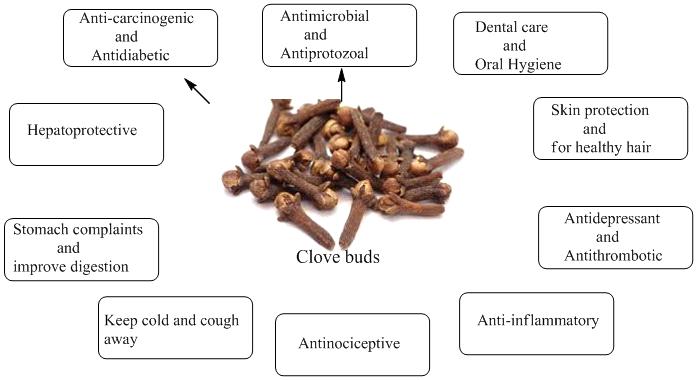
The clove exerts its analgesic effect by the activation of calcium and chloride channels in ganglion cells. The inhibition of voltage dependant calcium and sodium channel and receptors expressed in the trigeminal ganglion also contributed to the analgesic effect of clove. It is also used for asthma, allergy disorders, acne, scars, and rheumatoid arthritis, and it also showed antispasmodic and acaricidal effects toward Dermatophagoides pteronyssinus and D. farina [32].
Furthermore, the clove oil has shown antipyretic, aphrodisiac, appetizer, anxiolytic, antiemetic, hypnotic, decongestant, antiepileptic, myorelaxant, and expectorant properties, also, has a medicinal influence against trophic disorder.
Clove oil was also reported as an inhibitor of platelet aggregation and thromboxane synthesis induced by arachidonic acid and platelet-activating factor and may act as an antithrombotic agent.
Studies have also revealed that the clove supplementation can reduce the in vivo increased blood sugar level and the lipid peroxidation in diabetic rats by the reestablishment of the levels of the antioxidant enzyme [33].
Additionally, it was also revealed that the dietary cloves have antioxidative organ protective effects by reduction of in vivo hyperglycemia-induced tissue damages in the liver, cataract formation in the lens, and cardiac muscles in rats.
Clove essential oils have been reported to increase blood circulation and can reduce the risk of arterial sclerosis, cardiovascular disorders, and other disease associated with oxidative stress. Eugenol also demonstrated reversible, dose-related negative inotropic activity as well as vasodilator action in heart muscle and showed smooth muscle relaxant and hypotensive efficacy.
Interestingly, the cytotoxic and anti-carcinogenic and activities of the clove oil have been reported against leukemia, lung, breast, and colorectal cancer cells. Eugenol and dehydrodieugenol present in clove have been shown to stimulate human cancer cell death [34,35].
In foods and beverages, clove is used as a flavoring agent.
The clove is also used in the manufacturing of soaps, toothpaste, perfumes, cosmetics, and cigarettes. Clove cigarettes, also named kreteks, generally contain 20% to 40% ground clove and 60% to 80% tobacco. Eugenol acts like menthol to reduce the harshness of tobacco smoke.
Fig 2: Therapeutic uses of clove
Clove Side Effects & Toxicity
Clove is mostly safe in amounts commonly found in food by oral administration. The daily allowable human consumption of clove oil approved by the WHO Expert Committee on Food additives is 2.5 mg/kg body weight.
Application of clove oil or cream to the skin can sometimes cause burning and irritation of the skin and in some people even allergic dermatitis. Moreover, the repeated oral application may result in gingival damage and skin and mucous membrane irritation.
The eugenol constituent in cloves may theoretically increase the risk of bleeding by slowing blood clotting in some people who are concomitantly using herbs such as ginger, garlic, ginkgo, and white willow bark.
Similarly, patients taking antiplatelet agents such as aspirin, dipyridamole, clopidogrel, heparin, ticlopidine, and warfarin may also experience an increased risk of bleeding.
Inhaling smoke from clove cigarettes can cause side effects such as breathing problems and lung disease.
The use of clove in children needs to be in check as excessive use may lead to liver damage, seizures, and fluid imbalances.
The large doses are not advised for breastfeeding mothers and pregnant women as it may lead to inflammation [36,37].
To conclude, the clove represents a very fascinating plant with huge potential as a food preservative and as a rich source of antioxidant components. Pharmacologically, clove and its main constituents possess antioxidant, antimicrobial, analgesic, anti-inflammatory, anticancer, and anesthetic effects.
Moreover, the clove has also shown aphrodisiac, and antipyretic activities and have a vital role in dentistry. These proven biological activities recommend the future development of this medicinal plant for human and animal uses.
About the Author

Dr. Harmeet Kaur received her Bachelors in Pharmacy from Guru Nanak Dev University in Amritsar, India in 2000. Guru Nanak Dev University is a state owned university with an "A" grade nationally.
Dr. Kaur received her Masters in Medicinal Chemistry from the National Institute of Pharmaceutical Education and Research in 2002.
In 2015 Dr. Kaur was awarded her Ph.D in Pharmaceutical Sciences from Maharshi Dayanad University in Rohtak, India.
Dr. Kaur is presently a Senior Research Scientist at Maharshi Dayanand University in India.
Dr. Kaur has over 35 published Research papers concerning infectious diseases caused by yeasts, fungi, and bacteria using both prescription drugs and natural plant compounds. She has also performed many studies on cancer cells.
Of particular importance to us, is her multiple published experiments performed on Candida albicans and pathogenic bacteria using natural compounds. Because of this experience, we are thrilled to have her on the YeastInfectionAdvisor team.
Back to Herbal Yeast Infection Remedies
If you have any questions about clove or yeast infections in general, please feel free to contact us from the contact page of this website or see your doctor.
Dr. Kaur's Medical References
[1]. Wei WA. The research progress of ornamental plant Syringa. Territory & Natural Resources Study. 2013(3):34.
[2]. Batiha GE, Alkazmi LM, Wasef LG, Beshbishy AM, Nadwa EH, Rashwan EK. Syzygium aromaticum L.(Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules. 2020;10(2):202.
[3]. Jirovetz L, Buchbauer G, Stoilova I, Stoyanova A, Krastanov A, Schmidt E. Chemical composition and antioxidant properties of clove leaf essential oil. Journal of Agricultural and Food Chemistry. 2006;54(17):6303-7.
[4]. Nassar MI, Gaara AH, El-Ghorab AH, Farrag A, Shen H, Huq E, Mabry TJ. Chemical constituents of clove (Syzygium aromaticum, Fam. Myrtaceae) and their antioxidant activity. Revista Latinoamericana de Química. 2007;35(3):47.
[5]. Mytle N, Anderson GL, Doyle MP, Smith MA. Antimicrobial activity of clove (Syzgium aromaticum) oil in inhibiting Listeria monocytogenes on chicken frankfurters. Food Control. 2006 Feb 1;17(2):102-7.
[6]. Ogunwande IA, Olawore NO, Ekundayo O, Walker TM, Schmidt JM, Setzer WN. Studies on the essential oils composition, antibacterial and cytotoxicity of Eugenia uniflora L. International Journal of Aromatherapy. 2005;15(3):147-52.
[7]. Fu Y, Zu Y, Chen L, Shi X, Wang Z, Sun S, Efferth T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytotherapy Research. 2007;21(10):989-94.
[8]. Palombo EA, Semple SJ. Antibacterial activity of Australian plant extracts against methicillin‐resistant Staphylococcus aureus (MRSA) and vancomycin‐resistant enterococci (VRE). Journal of Basic Microbiology: An International Journal on Biochemistry, Physiology, Genetics, Morphology, and Ecology of Microorganisms. 2002;42(6):444-8.
[9]. Chaieb K, Hajlaoui H, Zmantar T, Kahla‐Nakbi AB, Rouabhia M, Mahdouani K, Bakhrouf A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2007;21(6):501-6.
[10]. Qiu J, Feng H, Lu J, Xiang H, Wang D, Dong J, Wang J, Wang X, Liu J, Deng X. Eugenol reduces the expression of virulence-related exoproteins in Staphylococcus aureus. Applied and Environmental Microbiology. 2010;76(17):5846-51.
[11]. Saeed S, Tariq P. In vitro antibacterial activity of clove against Gram-negative bacteria. Pakistan Journal of Botany 2008;40(5):2157-60.
[12]. Leite AM, Lima ED, Souza EL, Diniz MD, Trajano VN, Medeiros IA. Inhibitory effect of beta-pinene, alpha-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Revista Brasileira de Ciências Farmacêuticas. 2007;43(1):121-6.
[13]. Ali SM, Khan AA, Ahmed I, Musaddiq M, Ahmed KS, Polasa H, Rao LV, Habibullah CM, Sechi LA, Ahmed N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Annals of Clinical Microbiology and Antimicrobials. 2005;4(1):1-7.
[14]. Hemaiswarya S, Doble M. Synergistic interaction of eugenol with antibiotics against Gram- negative bacteria. Phytomedicine. 2009;16(11):997-1005.
[15]. Kunicka-Styczyńska A, Tyfa A, Laskowski D, Plucińska A, Rajkowska K, Kowal K. Clove Oil (Syzygium aromaticum L.) activity against Alicyclobacillus acidoterrestris biofilm on technical surfaces. Molecules. 2020;25(15):3334.
[16]. Cui H, Zhang C, Li C, Lin L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control. 2018;94:140-6.
[17]. Kim J, Marshall MR, Wei CI. Antibacterial activity of some essential oil components against five foodborne pathogens. Journal of agricultural and food chemistry. 1995;43(11):2839-45.
[18]. Latifah-Munirah B, Himratul-Aznita WH, Mohd Zain N. Eugenol, an essential oil of clove, causes disruption to the cell wall of Candida albicans (ATCC 14053). Frontiers in Life Science. 2015;8(3):231-40.
[19]. Eman-Abdeen E, El-Diasty EM. Antifungal activity of clove oil on dermatophytes and other fungi. International Journal of Advanced Research. 2015;3(12):1299-305.
[20]. Núñez L, D'aquino M, Chirife J. Antifungal properties of clove oil (Eugenia caryophylata) in sugar solution. Brazilian Journal of Microbiology. 2001;32(2):123-6.
[21]. Muñoz Castellanos L, Amaya Olivas N, Ayala-Soto J, De La O Contreras CM, Zermeño Ortega M, Sandoval Salas F, Hernández-Ochoa L. In Vitro and In Vivo Antifungal Activity of Clove (Eugenia caryophyllata) and Pepper (Piper nigrum L.) Essential Oils and Functional Extracts Against Fusarium oxysporum and Aspergillus niger in Tomato (Solanum lycopersicum L.). International Journal of Microbiology. 2020 May 1;2020.
[22]. Pinto E, Vale-Silva L, Cavaleiro C, Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. Journal of Medical Microbiology. 2009;58(11):1454-62.
[23]. Kaur K, Kaushal S, Rani R. Chemical composition, antioxidant and antifungal potential of clove (Syzygium aromaticum) essential oil, its major compound and its derivatives. Journal of Essential Oil Bearing Plants. 2019;22(5):1195-217.
[24]. Hussein G, Miyashiro H, Nakamura N, Hattori M, Kakiuchi N, Shimotohno K. Inhibitory effects of Sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2000;14(7):510-6.
[25]. Kurokawa M, Hozumi T, Basnet P, Nakano M, Kadota S, Namba T, Kawana T, Shiraki K. Purification and Characterization of Eugeniin as an Anti-herpesvirus Compound from Geum japonicum andSyzygium aromaticum. Journal of Pharmacology and Experimental Therapeutics. 1998 Feb 1;284(2):728-35.
[26]. Minami M, Kita M, Nakaya T, Yamamoto T, Kuriyama H, Imanishi J. The inhibitory effect of essential oils on herpes simplex virus type‐1 replication in vitro. Microbiology and Immunology. 2003;47(9):681-4.
[27]. Machado M, Dinis AM, Salgueiro L, Custódio JB, Cavaleiro C, Sousa MC. Anti-Giardia activity of Syzygium aromaticum essential oil and eugenol: effects on growth, viability, adherence and ultrastructure. Experimental Parasitology. 2011;127(4):732-9.
[28]. El-Kady AM, Ahmad AA, Hassan TM, El-Deek HE, Fouad SS, Althagfan SS. Eugenol, a potential schistosomicidal agent with anti-inflammatory and antifibrotic effects against Schistosoma mansoni, induced liver pathology. Infection and Drug Resistance. 2019;12:709.
[29]. https://www.thecandidadiet.com/cloves/
[30]. Diego CR, Wanderley OP. Clove (Syzygium aromaticum): a precious spice. Asian Pacific Journal of Tropical Biomedicine. 2014;(2):90-6.
[31]. Hussain S, Rahman R, Mushtaq A, El Zerey-Belaskri A. Clove: A review of a precious species with multiple uses. 2017; 11:129-133.
[32]. Rim IS, Jee CH. Acaricidal effects of herb essential oils against Dermatophagoides farinae and D. pteronyssinus (Acari: Pyroglyphidae) and qualitative analysis of a herb Mentha pulegium (pennyroyal). The Korean Journal of Parasitology. 2006;44(2):133.
[33]. Shukri R, Mohamed S, Mustapha NM. Cloves protect the heart, liver and lens of diabetic rats. Food Chemistry. 2010;122(4):1116-21.
[34]. Pisano M, Pagnan G, Loi M, Mura ME, Tilocca MG, Palmieri G, Fabbri D, Dettori MA, Delogu G, Ponzoni M, Rozzo C. Antiproliferative and pro-apoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Molecular Cancer. 2007;6(1):8.
[35]. Kumar PS, Febriyanti RM, Sofyan FF, Luftimas DE, Abdulah R. Anticancer potential of Syzygium aromaticum L. in MCF-7 human breast cancer cell lines. Pharmacognosy Research. 2014;6(4):350.
[36]. https://www.webmd.com/vitamins/ai/ingredientmono-251/clove
[37]. Sarrami N, Pemberton MN, Thornhill MH, Theaker ED. Adverse reactions associated with the use of eugenol in dentistry. British Dental Journal. 2002;193(5):257-9.
Home Privacy Policy Copyright Policy Disclosure Policy Doctors Store
Copyright © 2003 - 2025. All Rights Reserved under USC Title 17. Do not copy
content from the pages of this website without our expressed written consent.
To do so is Plagiarism, Not Fair Use, is Illegal, and a violation of the
The Digital Millennium Copyright Act of 1998.