- Home
- Species & Infections
- Candida Glabrata
Candida glabrata - What You Need to Know
Updated 5/12/2024
Written by Molecular Biologist Dr. Vibhuti Rana, PhD
Candida glabrata is currently the second/third most common causative agent for oral, esophageal, genital, or urinary tract yeast infections; not to forget the systemic hospital-acquired nosocomial infections (1). In Europe and the United States of America, Candida glabrata has been ranked as one of the most clinically significant fungal pathogens of genus Candida, after Candida albicans.
The genus Candida was discovered around late 1900s; before that C. glabrata was known as Torulopsis glabrata, and even before that, Cryptococcus glabrata (1917). The current taxonomy of C. glabrata is the Kingdom Fungi, Subkingdom Dikarya, Phlyum Ascomycota, Subphylum Saccharomycotina, Class Saccharomycetes, Order Saccharomycetales, Family Saccharomycetaceae, Genus Nakaseomyces, Clade Nakaseomyces/Candida and Species glabrata (NCBI:txid284593) (2). Candida glabrata has shown a greater evolutionary and genomic similarity with the non-pathogenic Saccharomyces cerevisiae rather than the pathogenic C. albicans (2). In fact, both Candida glabrata and Saccharomyces cerevisiae are believed to have evolved from the same tetraploid hybrid ancestor about 100 to 200 million years ago (3, 4)!
The salient characters of this emerging opportunistic pathogen include whitish/purplish colony appearance, haploid genome, lack of true hyphae, and some typical biochemical features. Interestingly, it was earlier thought to be a non-transforming yeast that simply lives saprophytically with normal microbes in the body; and was considered to be relatively non-pathogenic. With a gradual rise in the level of research done worldwide, discovery of pseudohyphal formation, phenotypic switching, and serious pathogenic conditions came to be associated with this species (5).
The Candida glabrata species complex is comprised of Candida glabrata sensu stricto, Candida nivariensis, and Candida bracarensis, which are difficult to separate from each other from common laboratory fungal detection methods; and have huge role to play in exerting invasive candidiasis (infections that invade your body internally) that shows highest mortality rate from fungal infections (6). These have now been identified using sophisticated gene sequencing and molecular technologies such as high resolution melting curve analysis. This situation of clinical invasive candidemia (Candida infection that reaches the blood stream) is further worsened due to the developing resistance of C. glabrata and its complex members towards azole class of drugs, especially fluconazole, the most common drug used to treat such infections (7).
How Does Candida glabrata Infect the Human Body?
Adherence is a key feature of C. glabrata. It is what helps the fungus to stick to the epithelial or macrophagic host cells and proceed with cell attachment, colonization, and ultimately, invasion. C. glabrata has more than 65 putative adhesin protein molecules which mediate adhesion to different types of host cells (8). In 1999, a study published in Science showed that deletion of EPA1 adhesin-encoding gene resulted in 95% reduction in its attachment to the human epithelial cells, highlighting the role of EPA genes in cell adhesion (9).
Next is their ability to form biofilms. Biofilms can be formed on biotic or abiotic surfaces and are complex structures formed as a result of inter-microbial or microbial-cell surface interactions taking place in the extracellular matrix. Biofilms are a crucial component of pathogenicity. C. glabrata shows dense biofilm formation which is rich in proteins and β-1, 3 glucan-containing carbohydrates (10). It has been known to develop biofilms on vascular and urinary catheters, leading to major treatment failures in case of C. glabrata and C. albicans infections. All this is done due to a regulatory interplay of a number of genes, including the ones encoding EPA6 and EPA7 adhesin proteins, which construct the biofilms. These biofilms have led to, and will continue to cause great negative effects on the therapeutic industry, because they make the cellular- or medical device-surfaces resistant to the anti-fungal drugs.
Candida albicans and Candida glabrata: How Similar and How Different?
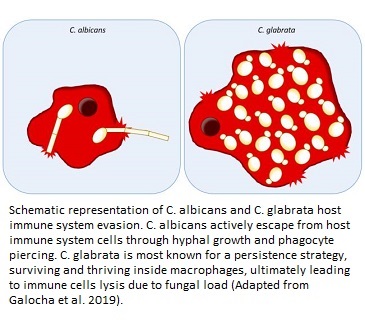
Both the species may show pathogenic traits in humans, but they are quite different as far as their genetics, functioning, mode of action, and phylogenetic features (ancestry) are concerned. While C. albicans has a diploid genome and has multiple pathogenic factors responsible for its virulence, C. glabrata has a haploid genome and has vaguely identified mechanisms of pathogenicity. Some of these mechanisms in the latter species could be pseudohyphal formation to reach host cells or accessing the bloodstream by adherence to medical devices, catheters, and via surgery. On the other hand C. albicans has proven cell penetration activity via hyphal growth, and induced endocytotic invasion of the fungus into the cells (11).
It is also interesting to understand the synergistic effect of both the pathogens. The degree of infection is much worse when C. glabrata invasion is accompanied by C. albicans infection in the host. This could be due to exploitation of host nutrients by C. glabrata with the help of C. albicans-mediated penetration and cell damage (12). In other words, C. albicans supports C. glabrata to carry out infection by paving way for it into the host cells. Once C. glabrata invades the blood stream, it also has an ability to get engulfed by the macrophages (the primary line of defense in blood infections), stay there, and replicate in them for their survival by chromatin remodeling. This is a few of the many complex ways by which it can evade host immune system and bring about life threatening infections (13).
In another study conducted by Brieland et al from the Schering Plough Research Institute, New Jersey, USA, it was found that C. glabrata leads to chronic non-fatal infection in immunocompetent mice models with induction of TNF-alpha, IL-12, and IFN-gamma proinflammatory cytokines. On the other hand, C. albicans caused fatal infection in the spleen, liver, and lungs of the mice and induction of IL-10 proinflammatory cytokine. However, in both cases of infection, TNF-alpha played a key role in defending the host cells from systemic candidiasis (14).
Whose at Risk for Candida glabrata Infection?
Under normal circumstances C. glabrata exists in harmony with the host cells in the mouth, intestine, and mucosa without any health threat. So the chances of developing this infection are low in normal individuals. However, they are heightened in individuals who have a weakened immunity; so, this infection marks the highest mortality rate in such people. These people can fall into different categories like those taking immunosuppressants, those who have undergone organ transplant surgeries, those with co-morbidities like cancer, HIV, and tuberculosis, diabetic people, those who have oral/dental complications and have to wear dentures (15, 16). In addition, elderly people and those patients in whom the gut microbial balance is disrupted due to frequent abuse of antibiotics are more likely to develop C. glabrata infections as compared to healthy individuals (16).
Implications of Candida glabrata Infections
Candida species have been ranked among the top three causes of hospital acquired blood stream infections, after Staphyloccus aureus and enterococci (17). Candida albicans and Candida glabrata account for more than 60% of the yeast species harboring the human body, and are known to cause more than 400,000 fatal infections globally every year (18). Moreover, rising cases of drug resistance, use of immunosuppressants, and systemic yeast infections have led to a corresponding rise in C. glabrata infections globally. It now dominates and causes serious infections in immunocompromised hosts, especially those with co-morbidities such as diabetes or HIV. Once it causes such high degree of infection in these set of patients, it is extremely challenging to treat them due to limited options (1, 19).
As we all can make out, different species of Candida would have different susceptibility patterns towards anti fungal therapies owing to their varying molecular mechanisms and genetic makeup. When compared with C. albicans infections affecting dental hygiene, the immune response triggered by C. glabrata in terms of pro-inflammatory cytokine production is lower. Cases of resistance towards antifungal activity of salivary histatins and mucins in the oral epithelial cells have also been reported widely, making C. glabrata a resilient fungal species to be tackled in case of overgrowth. It shows adaptive as well as innate resistance against different anti fungal drugs (20).
Modes of Treatment of Candida glabrata Infections
Fluconazole is the first choice of therapy when encountered with C. glabrata infections. However, rising resistance to this drug (almost 15% of C. glabrata isolates found in bloodstream exhibit resistance to prophylactic fluconazole) has led to finding other replacements of fluconazole. As of now, the systemic and vaginal infections have been studied in vivo using animal models in order to understand more about treatment, pathogenesis, and immunity. Treatment of C. glabrata infections can include azoles but often requires amphotericin B or flucytosine (21).
Another study by Olson et al., in 2005 showed the effect of using liposomal amphoterin C, echinocandin caspofungin, micafungin, or their combinations to treat immunosuppressed mice infected with the C. glabrata pathogen. What this study found was that infection was completely eradicated only when liposomal amphotericin B was given either along with caspofungin or micafungin or if it B was given consecutively after administering caspofungin. In other words, liposomal amphotericin and Echinocandins work hand in hand to enhance the treatment outcomes in such mice models (22). A broad-spectrum pneumocandin antifungal drug (L-743,872) has also been developed by Merck Research and is being evaluated in mouse models for its efficacy in C. glabrata infections (5).
Likewise, many other research groups across the globe are pitching together to find therapeutic remedies for C. glabrata systemic infections.
To learn how to treat Candida glabrata naturally click here.
About the Author

Dr. Vibhuti Rana completed her Bachelors's Degree (Bioinformatics Hons.) from Punjab University and accomplished her Master’s Degree (2012) in Genomics with a Gold Medal from Madurai Kamaraj University, India. In 2020, she received her doctorate in Molecular Biology from the Council of Scientific and Industrial Research-Institute of Microbial Technology in affiliation with the Jawaharlal Nehru University, New Delhi, India.
Her focus areas include microbial drug resistance, epidemiology, and protein-protein interactions in infectious diseases. As a Molecular Biologist with extensive experience with infectious diseases, we are happy she is part of the YeastInfectionAdvisor team.
Any questions about Candida glabrata or yeast infections in general? Please feel free to contact us from the contact page of this website or talk to your doctor.
Dr. Rana's Medical References
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC88907/
- Kumar K, Askari F, Sahu MS, Kaur R. Candida glabrata: A Lot More Than Meets the Eye. Microorganisms. 2019;7(2):39. Published 2019 Jan 30. doi:10.3390/microorganisms7020039. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6407134/
- Gabaldón T, Carreté L. The birth of a deadly yeast: tracing the evolutionary emergence of virulence traits in Candida glabrata. FEMS Yeast Res. 2016;16(2):fov110. doi:10.1093/femsyr/fov110. https://pubmed.ncbi.nlm.nih.gov/26684722/
- Gabaldón T, Martin T, Marcet-Houben M, et al. Comparative genomics of emerging pathogens in the Candida glabrata clade. BMC Genomics. 2013;14:623. Published 2013 Sep 14. doi:10.1186/1471-2164-14-623. https://pubmed.ncbi.nlm.nih.gov/24034898/
- Graybill JR, Bocanegra R, Luther M, Fothergill A, Rinaldi MJ. Treatment of murine Candida krusei or Candida glabrata infection with L-743,872. Antimicrobial Agents and Chemotherapy. 1997, 41 (9) 1937-1939; DOI: 10.1128/AAC.41.9.1937. https://aac.asm.org/content/41/9/1937.short
- Cai, S, Xu, J, Shao, Y, et al. Rapid identification of the Candida glabrata species complex by high‐resolution melting curve analysis. J Clin Lab Anal. 2020; 34:e23226. https://doi.org/10.1002/jcla.23226. https://onlinelibrary.wiley.com/action/showCitFormats?doi=10.1002%2Fjcla.23226
- Mirhendi H, Bruun B, Schønheyder HC, et al. Differentiation of Candida glabrata, C. nivariensis and C. bracarensis based on fragment length polymorphism of ITS1 and ITS2 and restriction fragment length polymorphism of ITS and D1/D2 regions in rDNA. Eur J Clin Microbiol Infect Dis. 2011;30(11):1409-1416. doi:10.1007/s10096-011-1235-9. https://pubmed.ncbi.nlm.nih.gov/21607825/
- de Groot PW, Kraneveld EA, Yin QY, et al. The cell wall of the human pathogen Candida glabrata: differential incorporation of novel adhesin-like wall proteins. Eukaryot Cell. 2008;7(11):1951-1964. doi:10.1128/EC.00284-08. https://pubmed.ncbi.nlm.nih.gov/18806209/
- Cormack BP, Ghori N, Falkow S. An Adhesin of the Yeast Pathogen Candida glabrata Mediating Adherence to Human Epithelial Cells. SCIENCE 1999: 578-582. https://science.sciencemag.org/content/285/5427/578.full
- d'Enfert C, Janbon G. Biofilm formation in Candida glabrata: What have we learnt from functional genomics approaches?. FEMS Yeast Res. 2016;16(1):fov111. doi:10.1093/femsyr/fov111. https://pubmed.ncbi.nlm.nih.gov/26678748/
- Galocha M, Pais P, Cavalheiro M, Pereira D, Viana R, Teixeira MC. Divergent Approaches to Virulence in C. albicans and C. glabrata: Two Sides of the Same Coin. Int J Mol Sci. 2019;20(9):2345. Published 2019 May 11. doi:10.3390/ijms20092345. https://pubmed.ncbi.nlm.nih.gov/31083555/
- Tati S, Davidow P, McCall A, Hwang-Wong E, Rojas IG, et al. (2016) Candida glabrata Binding to Candida albicans Hyphae Enables Its Development in Oropharyngeal Candidiasis. PLOS Pathogens 12(3): e1005522. https://doi.org/10.1371/journal.ppat.1005522. https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1005522
- Bolotin‐Fukuhara, M. and Fairhead, C. (2014) Candida glabrata: a deadly companion? Yeast 31: 279-288. doi:10.1002/yea.3019. https://onlinelibrary.wiley.com/doi/full/10.1002/yea.3019
- Brieland J, Essig D, Jackson C, et al. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect Immun. 2001;69(8):5046-5055. doi:10.1128/IAI.69.8.5046-5055.2001. https://pubmed.ncbi.nlm.nih.gov/11447185/
- Khatib R, Johnson LB, Fakih MG, Riederer K, Briski L. Current trends in candidemia and species distribution among adults: Candida glabrata surpasses C. albicans in diabetic patients and abdominal sources. Mycoses. 2016;59(12):781-786. doi:10.1111/myc.12531. https://pubmed.ncbi.nlm.nih.gov/27402377/
- Pfaller MA, Andes DR, Diekema DJ, et al. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004-2008. PLoS One. 2014;9(7):e101510. Published 2014 Jul 3. doi:10.1371/journal.pone.0101510. https://pubmed.ncbi.nlm.nih.gov/24991967/
- Banerjee SN, Emori TG, Culver DH, et al. Secular trends in nosocomial primary bloodstream infections in the United States, 1980-1989. National Nosocomial Infections Surveillance System. Am J Med. 1991;91(3B):86S-89S. doi:10.1016/0002-9343(91)90349-3. https://pubmed.ncbi.nlm.nih.gov/1928197/
- Olson ML, Jayaraman A, Kao KC. Relative Abundances of Candida albicans and Candida glabrata in In Vitro Coculture Biofilms Impact Biofilm Structure and Formation. Applied and Environmental Microbiology 2018, 84 (8) e02769-17; DOI: 10.1128/AEM.02769-17. https://aem.asm.org/content/84/8/e02769-17.short
- Sinnott JT 4th, Cullison JP, Sweeney MP. Candida (Torulopsis) glabrata. Infect Control. 1987;8(8):334-336. doi:10.1017/s0195941700066443. https://pubmed.ncbi.nlm.nih.gov/3308741/
- Li L, Redding S, Dongari-Bagtzoglou A. Candida glabrata: an emerging oral opportunistic pathogen. J Dent Res. 2007;86(3):204-215. doi:10.1177/154405910708600304. https://journals.sagepub.com/doi/abs/10.1177/154405910708600304).
- Farmakiotis D, Tarrand JJ, Kontoyiannis DP. Drug-resistant Candida glabrata infection in cancer patients. Emerg Infect Dis. 2014;20(11):1833-1840. doi:10.3201/eid2011.140685. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4214312/).
- Olson JA, Adler-Moore JP, Smith PJ, Proffitt RT. Treatment of Candida glabrata infection in immunosuppressed mice by using a combination of liposomal amphotericin B with caspofungin or micafungin. Antimicrob Agents Chemother. 2005;49(12):4895-4902. doi:10.1128/AAC.49.12.4895-4902.2005. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1315958/
Home Privacy Policy Copyright Policy Disclosure Policy Doctors Store
Copyright © 2003 - 2025. All Rights Reserved under USC Title 17. Do not copy
content from the pages of this website without our expressed written consent.
To do so is Plagiarism, Not Fair Use, is Illegal, and a violation of the
The Digital Millennium Copyright Act of 1998.