- Home
- Species & Infections
- Cryptococcus Neoformans
Cryptococcus neoformans - A Pathogenic Budding Yeast
Posted 6/15/2025
Written by Molecular Biologist Dr. Vibhuti Rana, PhD
Cryptococcus neoformans is a unicellular budding yeast species that is found in the natural environment like soil, tree hollows, and bird excreta. It mostly affects people in the tropical and sub tropical regions like sub Saharan Africa and South East Asian developing countries, where it is the most common cause of meningitis. It is most commonly associated with meningo-encephalitis-an inflammatory infection of the brain and its surrounding regions.
This infection is all the more pronounced in patients with co-existing HIV and AIDS conditions (roughly 1 million cases every year, out of which 0.6 million people die as early as within three months of infection), as reported in a study published by Park et al. in 2018 (1).
It is also known to invade the human lungs upon inhalation and displays pneumonia-like symptoms. It is the lungs from where the pathogen travels to the brain to cause meningitis.
About Cryptococcus neoformans
Cryptococcus neoformans belongs to the fungal phylum Basidiomycota-known to infect mammalian hosts. Two species- C. neoformans and C. gattii (a C. neoformans strain producing elongated cells)-are a part of the C. neoformans complex. These pathogens have been known to diverge nearly 34 million years ago and are marked by a number of different traits. For example, DNA amplification studies carried out in the Australian geography suggests that C. neoformans has worldwide distribution, is found in bird excreta, and mainly causes human infections. On the contrary, C. gattii is responsible for human infections with a much lesser frequency, found in the tropical/ sub-tropical regions, and is associated with eucalyptus trees (2-4).
If we go into the history of discovery of this pathogen, it was first isolated in 1894 from the infected bones of a woman in her prime, and termed as a Saccharomyces- like yeast organism (5, 6). In the same year, it was isolated from peach juice and named Saccharomyces neoformans and finally; it was renamed Cryptococcus neoformans due to lack of forming ascospores (the typical nature of genus Saccharomyces). It is also known by some other synonyms like Filobasidiella neoformans, Filobasidiella bacillispora, Cryptococcus bacillispora, Cryptococcus, etc. (7,8). Barnett et al also found a link between the rise in AIDS cases and infections by this rare opportunistic pathogen (8).
Generally, this fungus stays in the haploid asexual state in the environment. Discovery of the infective hyphal form in 1966 and sexual reproduction in C. neoformans in 1975 gradually initiated the exploitation of genetic manipulation and genomic understanding of the pathogen (6, 7). A number of nutritional cues can lead to stimulation of sexual morphology of C. neoformans. Studies show that myo-inositol, copper ions, and nitrogen starvation. Mating was also supported on plant surfaces, as seen experimentally in case of Arabidopsis thaliana and Eucalyptus camaldulensis (9).
Pigeon droppings specifically supported the propagation of C. neoformans and not C. gattii. C. neoformans is mesophilic and being thermotolerant, can sustain itself at a much lower environmental temperature as compared to the host body temperatures. This is because of the expression of special temperature regulating genes in the cryptococcal genome (10).
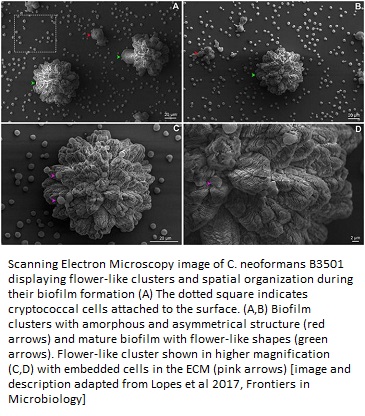
A collaborative study between Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil and Massachusetts Institute of Technology, Cambridge, beautifully captured the geometrical stages of the C. neoformans biofilm formation at its adhesion step, which makes it resilient against drug treatment. These extracellular matrix-embedded cells were visualized using scanning electron microscopy (11).
In a review article published in Microorganisms in 2020, Maliehe et al talk about the environmental cues that decide the pathogenesis in the C. neoformans model. In this very interesting piece of information, they explore if C. neoformans, which is originally an environmental pathogen, loses its pathogenicity upon re-entering the environment (by decomposition of the infected host) or stays virulent with the help of environmental factors (12).
Turns out that C. neoformans exploits the environmental stress conditions to its advantage and produces virulent factors. It also regulates the cyclic AMP/protein kinase A, calcium/calcineurin dependent/independent calmodulin cell signaling pathways for promoting Cryptococcus mediated virulence and thermo tolerance, high CO2 resistance, alkaline pH resistance, cell wall integrity and architecture, and cell mating (13).
How Does C. neoformans Affect the Human Body?
This fungal species has the ability to switch between the budding vegetative yeast form and the multicellular sexual hyphal forms. It is the spores which are released in this sexual form that impart pathogenicity or infectious nature to the pathogen inside the lungs. The sexual form also renders greater genetic variability to the pathogen, resulting in highly virulent strains (14).
These spores are infectious and when they enter the blood stream, cross the blood-brain-barrier and colonize in the brain tissues. Upon inhalation, the spores can also colonize and infect the lungs of immuno-compromised individuals. Fortunately, this species is unable to transmit from the infected individual to a healthy individual (like Mycobacterium tuberculosis bacterium and SARS-CoV2 virus which spread via aerosol inhalation and fomites) and controls the propagation of the fungus (12).
While in vitro results suggest that this pathogen behaves dormant and causes minimal damage to the cellular structures, a leading review published recently in the Frontiers in Immunology by Casadevall et al. suggests otherwise. It concludes that this fungal pathogen is indirectly able to damage the mammalian host cells and tissues and when this damage is enough to cause an imbalance in the host system, the whole organism gets infected. The mechanisms most likely involved in this whole cascade include lytic exocytosis, organelle dysfunction, phago-lysosomal membrane damage, and cytoskeletal alterations (15)-all of which lead to a disruption of the immune cell function and dissemination of the pathogen.
The main steps in the induction of the polysaccharide capsule, the most important virulence factor of C. neoformans infection, are biosynthesis, transport, and maintenance of the polysaccharide at its cell surface. This capsule is uniquely formed depending upon the host-environment conditions; and the virulence comprises of complicated signaling pathways and molecules rendering chronic infection (16).
Secondly, the Cryptococcus enzyme phenoloxidase, which synthesizes the pigment melanin (responsible for imparting color/pigmentation in the skin) helps this fungal pathogen to stay immune against the host defense system and serves as an important virulence factor (17).
Casadevall et al. discuss a holistic approach wherein the damage caused by C. neoformans can be classified into four levels: molecular, cellular, tissue, and organism level. If these all occur together, Cryptococcus infection is exhibited in its worst form. At the molecular level, proteases, nuclease, urease, phospholipases of the fungus cause damage to host defense molecules. Next, at the cellular level, a lot of dysfunction occurs, e.g., faulty phagolysomal maturation, accumulation of polysaccharide vesicles, fragmentation of mitochondria, initiation of cell death pathways, and exocytosis. Tissue organization is disrupted and accumulation of the fungal masses occurs at the tissue level. Finally, the worst outcomes of this infection like nervous damage, hypertension, and fatality are visible at the organism level, as clearly seen in cats, and mice models (15).
Target Population of C. neoformans Infection
Similar to other fungal infections, C. neoformans succeeds in infecting the host only when the host immune system is weakened. The reasons of this compromised immunity could possibly range from co-existence of HIV-AIDS, organ transplant recipients, or people taking immunosuppressive agents in case of autoimmune diseases. The infection may stay latent or dormant till it finds a suitable opportunity to attack and overpower the host immune system. Therefore, it is important to catch even the latent infection in AIDS patients for timely treatment.
The major sites for infection by C. neoformans and C. gatti in humans are lungs and the central nervous system. However, other less common sites include the skin, prostrate, joints, eyes, and bones. When C. neoformans affects the lungs, symptoms like cough, fever, congestion, chest pain are seen. On the other hand, during meningoencephalitis, the infection moves from the lungs to the brain and is presented by fever, headaches, neck pains, vomiting, or nausea. In worst conditions, it leads to mental trauma and coma (18).
Diagnostics and Treatment
Targeted screening is commonly employed in HIV AIDS patients before they begin with antiretroviral therapy. This is done by simply detecting a cryptococcal antigenic protein via blood test. If the test comes positive, it indicates silent C. neoformans infection, which ought to be treated with antifungals like fluconazole.
A reasonable “Dipstick” test has been introduced by the Centers for Disease Control and Infection (CDC) to detect cryptococcal meningitis 95% of the times, even in advanced stages.
Anti-mycotics including Amphotericin B and flucytosine have been used to enhance the life of patients showing advanced cryptococcus. For HIV-AIDS patients, a two-week induction phase and an eight-week consolidation phase using amphotericin B and flucytosine are being used, in accordance with the common World Health Organization guidelines. As a maintenance treatment, fluconazole is given subsequently (18).
By now, you must have realized how important it is for the scientific community to formulate novel global strategies for prevention and treatment of Cryptococcus infection in order to understand its burden.
About the Author

Dr. Vibhuti Rana completed her Bachelors's Degree (Bioinformatics Hons.) from Punjab University and accomplished her Master’s Degree (2012) in Genomics with a Gold Medal from Madurai Kamaraj University, India. In 2020, she received her doctorate in Molecular Biology from the Council of Scientific and Industrial Research-Institute of Microbial Technology in affiliation with the Jawaharlal Nehru University, New Delhi, India.
Her focus areas include microbial drug resistance, epidemiology, and protein-protein interactions in infectious diseases. As a Molecular Biologist with extensive experience with infectious diseases, we are happy she is part of the YeastInfectionAdvisor team.
Any questions about Cryptococcus
neoformans or yeast infections in general, please feel free to contact us from the contact page of this website or talk to your doctor.
Dr. Rana's Medical References
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009 Feb 20;23(4):525-30. doi: 10.1097/QAD.0b013e328322ffac. PMID: 19182676. https://pubmed.ncbi.nlm.nih.gov/19182676/
- Ellis DH, Pfeiffer TJ. Natural habitat of Cryptococcus neoformans var. gattii. J Clin Microbiol. 1990 Jul;28(7):1642-4. doi: 10.1128/JCM.28.7.1642-1644.1990. PMID: 2199524; PMCID: PMC268004. https://pubmed.ncbi.nlm.nih.gov/2199524/
- Sorrell TC, Brownlee AG, Ruma P, Malik R, Pfeiffer TJ, Ellis DH. Natural environmental sources of Cryptococcus neoformans var. gattii. J Clin Microbiol. 1996 May;34(5):1261-3. doi: 10.1128/JCM.34.5.1261-1263.1996. PMID: 8727913; PMCID: PMC228992.https://pubmed.ncbi.nlm.nih.gov/8727913/
- Kwon-Chung KJ, Fraser JA, Doering TL, Wang Z, Janbon G, Idnurm A, Bahn YS. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb Perspect Med. 2014 Jul 1;4(7):a019760. doi: 10.1101/cshperspect.a019760. PMID: 24985132; PMCID: PMC4066639. https://pubmed.ncbi.nlm.nih.gov/24985132/
- Busse O. Uber parasitare Zelleinschlusse und ihre Zuchtung. Centralbl. Bakt. Parasit. 1894;16.
- Srikanta D, Santiago-Tirado FH, Doering TL. Cryptococcus neoformans: historical curiosity to modern pathogen. Yeast. 2014;31(2):47-60. doi:10.1002/yea.2997. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3938112/
- Kwon-Chung KJ. A new genus, filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia. 1975 Nov-Dec;67(6):1197-200. PMID: 765816. https://pubmed.ncbi.nlm.nih.gov/765816/
- Barnett JA. A history of research on yeasts 14: medical yeasts part 2, Cryptococcus neoformans. Yeast. 2010 Nov;27(11):875-904. doi: 10.1002/yea.1786. https://onlinelibrary.wiley.com/doi/full/10.1002/yea.1786
- Xue C, Tada Y, Dong X, Heitman J. The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host Microbe. 2007 Jun 14;1(4):263-73. doi: 10.1016/j.chom.2007.05.005. PMID: 18005707. https://pubmed.ncbi.nlm.nih.gov/18005707/
- Guijarro JA, Cascales D, García-Torrico AI, García-Domínguez M, Méndez J. Temperature-dependent expression of virulence genes in fish-pathogenic bacteria. Front Microbiol. 2015 Jul 9;6:700. doi: 10.3389/fmicb.2015.00700. PMID: 26217329; PMCID: PMC4496569. https://pubmed.ncbi.nlm.nih.gov/26217329/
- Lopes W, Vainstein MH, De Sousa Araujo GR, Frases S, Staats CC, de Almeida RMC, Schrank A, Kmetzsch L, Vainstein MH. Geometrical Distribution of Cryptococcus neoformans Mediates Flower-Like Biofilm Development. Front Microbiol. 2017 Dec 19;8:2534. doi: 10.3389/fmicb.2017.02534. PMID: 29312225; PMCID: PMC5742216. https://pubmed.ncbi.nlm.nih.gov/29312225/
- Maliehe M, Ntoi MA, Lahiri S, Folorunso OS, Ogundeji AO, Pohl CH, Sebolai OM. Environmental Factors That Contribute to the Maintenance of Cryptococcus neoformans Pathogenesis. Microorganisms. 2020 Jan 28;8(2):180. doi: 10.3390/microorganisms8020180. PMID: 32012843; PMCID: PMC7074686. https://pubmed.ncbi.nlm.nih.gov/32012843/
- Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997 May 15;16(10):2576-89. doi: 10.1093/emboj/16.10.2576. PMID: 9184205; PMCID: PMC1169869. https://pubmed.ncbi.nlm.nih.gov/9184205/
- Kozubowski L, Heitman J. Profiling a killer, the development of Cryptococcus neoformans. FEMS Microbiol Rev. 2012 Jan;36(1):78-94. doi: 10.1111/j.1574-6976.2011.00286.x. Epub 2011 Jul 4. PMID: 21658085; PMCID: PMC3318972. https://pubmed.ncbi.nlm.nih.gov/21658085/
- Casadevall A, Coelho C, Alanio A. Mechanisms of Cryptococcus neoformans-Mediated Host Damage. Front Immunol. 2018;9:855. Published 2018 Apr 30. doi:10.3389/fimmu.2018.00855. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5936990/
- O'Meara TR, Alspaugh JA. The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev. 2012 Jul;25(3):387-408. doi: 10.1128/CMR.00001-12. PMID: 22763631; PMCID: PMC3416491. https://pubmed.ncbi.nlm.nih.gov/22763631/
- Wang Y, Aisen P, Casadevall A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun. 1995;63(8):3131-3136. doi:10.1128/IAI.63.8.3131-3136.1995. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC173427/
- https://www.cdc.gov/fungal/pdf/at-a-glance-508c.pdf
Home Privacy Policy Copyright Policy Disclosure Policy Doctors Store
Copyright © 2003 - 2025. All Rights Reserved under USC Title 17. Do not copy
content from the pages of this website without our expressed written consent.
To do so is Plagiarism, Not Fair Use, is Illegal, and a violation of the
The Digital Millennium Copyright Act of 1998.