- Home
- Species & Infections
- Candida Tropicalis
Candida tropicalis - Second Only to Candida albicans
Updated 10/14/2021
Written by Molecular Biologist Dr. Vibhuti Rana, PhD
After Candida albicans, the non-C. albicans Candida yeast species- Candida tropicalis has emerged to be a potential cause of fungal yeast infections which include candidiasis, candidemia, or fungemia. The other key members of the non-C. albicans Candida species that have been implicated in fungal infections in humans include C. parapsilosis, C. krusei, C. tropicalis, and C. glabrata. Even though C. tropicalis is less prevalent than C. albicans in most parts of the world, it is still a huge contributor to human infections; particularly ascribing to the high mortality rate in patients infected by C. tropicalis. The prevalence of C. tropicalis varies from 3% to 66%, with a higher distribution in tropical and sub tropical regions of the world (1).
Here’s an interesting fact about C. tropicalis: It has wide application in the olive oil industry; in degradation of olive-tree pruning biomass for biodiesel production. In 2020, a review published in the journal Processes speaks about the fermentation of the olive oil industry byproducts and use of yeasts for generating energy (2). The olive-oil production results in byproducts and olive pruning debris rich in cellulose, hemicellulose, and lignin. The fermentation of hemicellulose component in the olive-tree prune is mediated by C. tropicalis and has great use in production of ethanol and xylitol by fermentation from the olive pruning (3). Additionally, C. tropicalis has promising use in biotechnological processes from other sources such as the production of xylitol from corn fibers and the ethanol from marine algae (4).
Historically speaking, Aldo Castellani first identified C. tropicalis in 1910 and named it as Oidium tropicale. This yeast species is also known by many other synonyms such as Monilia tropicalis, Candida vulgaris, Mycotorula dimorpha, and Candida paratropicalis. Finally, in 1920s, it was finally decided to be named and recorded as Candida tropicalis (5).
This opportunistic human pathogen is a diploid ascomycete yeast and is known to reside in a number of different sites of the human body. In a study conducted at the V.P. Chest Institute of Delhi in 2003 in around 85 clinical specimens isolated from more than 350 different sources, it was found that C. tropicalis colonizes multiple sites such as the skin, gastrointestinal tract, genitourinary tracts, and sometimes the respiratory tract (6). In addition, many reports show its existence and dissemination from non living surfaces and abiotic settings like medical devices (7).
If you are wondering how C. tropicalis infections spread, there are two answers. In the first case, a dysbiosis in the normal human microflora (altered or imbalance microflora) may result in translocation and spreading of this yeast (which was peacefully residing with other bacteria and fungi) through the gut to other sites. Apart from spreading endogenously, it can spread by hands of medical staff in hospital settings via the usage of prosthetics, catheters, parenteral administration of drugs, etc.
Phenotypically observed colonies of C. tropicalis on the Sabouraud dextrose agar (SDA) are cream-colored with a slightly mycelial border. These colonies, however, exhibit darkish blue color on CHROMagar (5). C. tropicalis is also capable of fermenting glucose, sucrose, galactose, trehalose, and maltose, but not lactose or rafkose.
C. albicans and C. tropicalis show a common genetic feature in terms of their genomic sequence: the presence of the major repeat sequence (MRS) elements, which may contribute to a similar virulence. This is not the case in C. parapsilosis and C. glabrata (8).
Candida yeast species generally live in harmony as a part of the human microbiome, following peaceful coexistence. However, as soon as an imbalance of microflora occurs, wherein the so called bad bacteria overpower the good soldiers, the host immunity gets compromised. That is when friendly Candida yeasts become ‘unfriendly’ and leads to a myriad of infections, both, local or systemic, including Candidemia (9). In most cases, this yeast species shows a very high dissemination in the neutropenic hosts and such infections prove to be fatal and are not diagnosed in a timely and effective fashion.
C. tropicalis has been implicated in high numbers in Urinary Tract Infections (UTIs), involving vaginal candidiasis. Some cases of oral candidiasis are also known to occur due to C. tropicalis invasions of mucosal epithelial tissues. It has been mostly isolated from the urinary tract (patients with candiduria) or blood stream (patients with candidemia) of cancer patients in the intensive care units (ICU) due to the high risk of (hospital acquired) nosocomial infections (10).
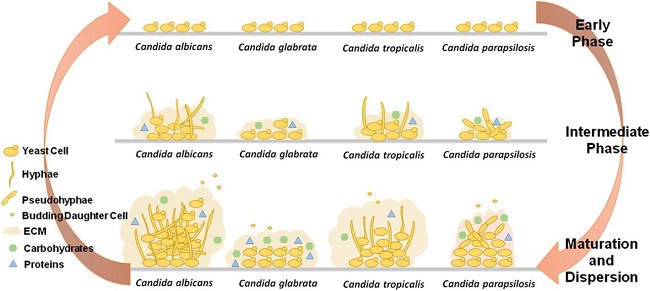
Differential capabilities of biofilm formation by Candida albicans, Candida glabrata, Candida tropicalis, and Candida parapsilosis- the main pathogenic species of Candida; each of these species has a variable potential to produce extracellular matrix (ECM) and its components based on the changes taking place in their cellular morphologies (image adapted from Cavalheiro and Teixeira, Front Med, 2018)
Owing to a similar nature to C. albicans, the chief mode of action of C. tropicalis is through the formation of biofilms. Not only do these biofilms protect the fungi from various antifungal drugs administered to the host, they also shield the pathogens from the host immune responses. The biofilm layers can be formed on cellular surfaces or abiotic surfaces like medical devices (catheters, defibrillators, joint replacements, prosthetic devices, etc.) as a result of inter-microbial or microbial-cell surface interactions that occur in the ECM. Most of the times, it is a mixture of bacterial and fungal pathogenic biofilms, making them all the more complex to eradicate.
C. tropicalis biofilm is presented by a combination of connectivity by the yeast, pseudohyphal, and hyphal forms, in addition to intense hyphal budding (11). The three main steps of biofilm formation are adhesion, proliferation, and maturation, which were seen to be established within 24-48 hours in case of C. tropicalis using scanning electron microscopy (11).
The biofilm production shown by C. tropicalis is almost as intense as compared to C. albicans, while this is not the case for C. glabrata and C. parapsilosis. This also depends on the type of available surfaces, the source of carbon available to the yeast, and the activity of different pathogenic enzymes (12). The extracellular polymeric substances present in the Candida biofilms regulate the susceptibility towards drug treatment by overexpression of drug efflux pump genes. These pumps basically throw out (expel) the drugs being given to the host, causing resistance and act as a barrier between the pathogen residing in the host cells and the external environment.
The other means by which it exerts its virulence are production of lytic enzymes such as proteinases, phospholipases, hydrolases, and hemolysins. Besides, this species can undergo phenotypic switching and morphogenesis- turning from non pathogenic budding form to the virulent hyphal form which penetrates and invades host cells (13). C. tropicalis has also been reported to be tolerant to extreme osmotic conditions (osmotolerant microorganism), helping it to face high salt concentrations and persist strongly in saline conditions.
Presentation of Infection by C. tropicalis and C. albicans
A study conducted in 2001 compared the fungemia cases of C. tropicalis and C. albicans amongst 43 and 148 cases of infected individuals, respectively. Consequently, it was found that leukemia (cancer of blood) and prolonged neutropenia (low level of white blood cells, mainly neutrophils) were the main risk factors for C. tropicalis infection in comparison to C. albicans. The same study did not suspect any significant differences in terms of drug response and catheter-associated infections among the two Candida species (14).
C. tropicalis is great at taking advantage of the immunocompromised host situation, even better than C. albicans. This was proven in animal models of neutropenia and mucositis upon introduction of both the pathogens (C. albicans and C. tropicalis isolates from patients with acute leukemia) in their gastrointestinal tracts (15). This study found that 20% of mice died upon inducing infection in case of C. tropicalis in comparison to only 4% for C. albicans infected blood isolates.
Therefore, it can be safely said that the factors responsible for gastrointestinal colonization, systemic dissemination, and mortality in immunocompromised hosts (especially those suffering from neutropenia and leukemia) are exerted differently in case of C. albicans and C. tropicalis.
Treating Candida tropicalis Allopathicly
As a result of the growing cases of fungal infections caused by Candida yeast species, it is indispensable to find effective therapeutic strategies for treating the target patient populations. This goal can be reached by consistent and in depth efforts to study all available modes of virulence of Candida like secretory enzymes and biofilm formation.
Timely recognition of Candida species greatly brightens the chances of survival in infected individuals and is required for designing the most suitable antifungal treatment. Modern techniques have enabled researchers to correctly identify C. tropicalis clinical strains from other medically important Candida species, even from the mixture of other microbes. These methods include molecular techniques like polymerase chain reaction (PCR) and realtime PCR assays involving melting curve analysis (16).
Invasive candidiasis or fungemia of blood resulting from C. tropicalis can be primarily treated by amphotericin B or an echinocandin, in addition to extended-spectrum triazole antifungals. Although preventive therapy using fluconazole has shown a decline in the occurrence of C. tropicalis-mediated candidosis, moderate level of fluconazole resistance has also been observed, probably resulting from a prolonged exposure or misuse of this azole drug (1). Very recently, a study in Algeria has also reported C. tropicalis to be the major contributor of mortality in candidemia-affected patients and the Egr11p protein showed amino acid mutations in the azole-resistant strains of C. tropicalis (17).
To sum it all up, active measures are called for developing successful antifungal therapeutics and prevent the prevalence of fungal infections due to Candida worldwide.
About the Author

Dr. Vibhuti Rana, PhD completed her Bachelors's Degree (Bioinformatics
Hons.) from Punjab University and accomplished her Master’s Degree
(2012) in Genomics with a Gold Medal from Madurai Kamaraj University,
India. In 2020, she received her doctorate in Molecular Biology from the
Council of Scientific and Industrial Research-Institute of Microbial
Technology in affiliation with the Jawaharlal Nehru University, New
Delhi, India.
Her focus areas include microbial drug resistance, epidemiology, and protein-protein interactions in infectious diseases. As a Molecular Biologist with extensive experience with infectious diseases, we are happy she is part of the YeastInfectionAdvisor team.
Any questions about Candida tropicalis or yeast infections in general, please contact us from the contact page of this website or talk to your doctor.
Dr. Rana's Medical References
- Chai LY, Denning DW, Warn P. Candida tropicalis in human disease. Crit Rev Microbiol. 2010; 36(4):282-298. doi:10.3109/1040841X.2010.489506. https://pubmed.ncbi.nlm.nih.gov/20883082/
- García Martín JF, Cuevas M, Feng C, Mateos PA,García MT and Sánchez S. Energetic Valorisation of Olive Biomass: Olive-Tree Pruning, Olive Stones and Pomaces. Processes 2020, 8(5), 511; https://doi.org/10.3390/pr8050511. https://www.mdpi.com/2227-9717/8/5/511/htm#
- Mateo S, Puentes JG, Moya AJ, Sánchez S. Ethanol and xylitol production by fermentation of acid hydrolysate from olive pruning with Candida tropicalis NBRC 0618. Bioresour Technol. 2015;190:1-6. doi:10.1016/j.biortech.2015.04.045. https://pubmed.ncbi.nlm.nih.gov/25916261/
- Rao RS, Jyothi ChP, Prakasham RS, Sarma PN, Rao LV. Xylitol production from corn fiber and sugarcane bagasse hydrolysates by Candida tropicalis. Bioresour Technol. 2006; 97(15):1974-1978. doi:10.1016/j.biortech.2005.08.015. https://pubmed.ncbi.nlm.nih.gov/16242318/
- Larone D (2002) Medically important fungi; a guide to identification, 4th edn. ASM Press, Washington. https://lib.ugent.be/en/catalog/rug01:000778429
- Basu S, Gugnani HC, Joshi S, Gupta N. Distribution of Candida species in different clinical sources in Delhi, India, and proteinase and phospholipase activity of Candida albicans isolates. Rev Iberoam Micol. 2003; 20(4):137-140. https://pubmed.ncbi.nlm.nih.gov/15456350/
- Negri M, Martins M, Henriques M, Svidzinski TI, Azeredo J, Oliveira R. Examination of potential virulence factors of Candida tropicalis clinical isolates from hospitalized patients. Mycopathologia. 2010; 169(3):175-182. doi:10.1007/s11046-009-9246-0. https://pubmed.ncbi.nlm.nih.gov/19851885/
- Butler et al. 2009. Nature 459 (7247) https://experts.illinois.edu/en/publications/evolution-of-pathogenicity-and-sexual-reproduction-in-eight-candi
- Kothavade RJ, Kura MM, Valand AG, Panthaki MH. Candida tropicalis: its prevalence, pathogenicity and increasing resistance to fluconazole. J Med Microbiol. 2010; 59(Pt 8):873-880. doi:10.1099/jmm.0.013227-0. https://pubmed.ncbi.nlm.nih.gov/20413622/
- Negri M, Silva S, Henriques M, Oliveira R. Insights into Candida tropicalis nosocomial infections and virulence factors. Eur J Clin Microbiol Infect Dis. 2012; 31(7):1399-1412. doi:10.1007/s10096-011-1455-z. https://pubmed.ncbi.nlm.nih.gov/22037823/
- Bizerra FC, Nakamura CV, de Poersch C, et al. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 2008;8(3):442-450. doi:10.1111/j.1567-1364.2007.00347.x. https://pubmed.ncbi.nlm.nih.gov/18248413/
- Cavalheiro M, Teixeira MC. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front Med (Lausanne). 2018; 5:28. Published 2018 Feb 13. doi:10.3389/fmed.2018.00028. https://pubmed.ncbi.nlm.nih.gov/29487851/
- Zuza-Alves DL, Silva-Rocha WP, Chaves GM. An Update on Candida tropicalis Based on Basic and Clinical Approaches. Front Microbiol. 2017;8:1927. Published 2017 Oct 13. doi:10.3389/fmicb.2017.01927. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5645804/
- Kontoyiannis DP, Vaziri I, Hanna HA et al. Risk Factors for Candida tropicalis Fungemia in Patients with Cancer, Clinical Infectious Diseases, Volume 33, Issue 10, 15 November 2001, Pages 1676–1681, https://doi.org/10.1086/323812. https://academic.oup.com/cid/article/33/10/1676/390288
- de Repentigny L, Phaneuf M, Mathieu LG. Gastrointestinal colonization and systemic dissemination by Candida albicans and Candida tropicalis in intact and immunocompromised mice. Infect Immun. 1992;60(11):4907-4914. doi:10.1128/IAI.60.11.4907-4914.1992. https://pubmed.ncbi.nlm.nih.gov/1399001/
- Fricke S, Fricke C, Schimmelpfennig C, et al. A real-time PCR assay for the differentiation of Candida species. J Appl Microbiol. 2010;109(4):1150-1158. doi:10.1111/j.1365-2672.2010.04736.x. https://pubmed.ncbi.nlm.nih.gov/20456528/
- Megri, Y., Arastehfar, A., Boekhout, T. et al.Candida tropicalis is the most prevalent yeast species causing candidemia in Algeria: the urgent need for antifungal stewardship and infection control measures. Antimicrob Resist Infect Control9, 50 (2020). https://doi.org/10.1186/s13756-020-00710-z.
Home Privacy Policy Copyright Policy Disclosure Policy Doctors Store
Copyright © 2003 - 2024. All Rights Reserved under USC Title 17. Do not copy
content from the pages of this website without our expressed written consent.
To do so is Plagiarism, Not Fair Use, is Illegal, and a violation of the
The Digital Millennium Copyright Act of 1998.